COVID-19: First Clinical Trial of Vaccine in US
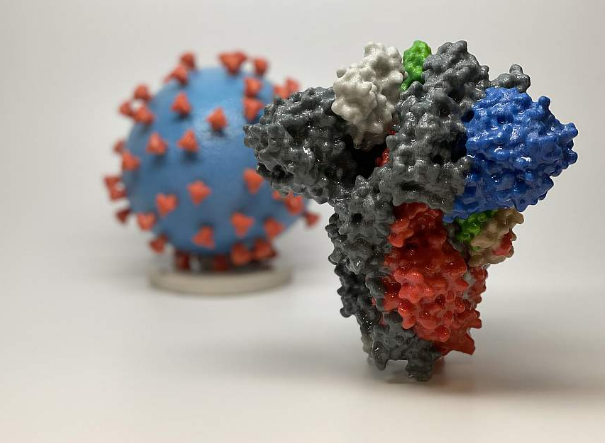
3D print of a spike protein of SARS-CoV-2—also known as 2019-nCoV, the virus that causes COVID-19—in front of a 3D print of a SARS-CoV-2 virus particle. The spike protein (foreground) enables the virus to enter and infect human cells. On the virus model,
Trial of an experimental vaccine against SARS-CoV-2 virus, the causative agent of COVID-19, began in the US on Monday. The phase 1 clinical trial has been conducted at Kaiser Permanente Washington Health Research Institute (KPWHRI) in Seattle. The trial has been funded by National Institute of Allergy and Infectious Diseases (NIAID), which is a part of the US-based premier research institute, the National Institute of Health (NIH).
The open-label trial will enroll 45 adult healthy individuals who have agreed to volunteer and will last for six weeks. The volunteers will be of the age ranging between 18 and 55 years. The study participants will receive two doses of the vaccine in the form of intramuscular injection in their upper arm, with a gap of approximately 28 days between the doses. Each volunteer will be given a 25 microgram (mcg), 100 mcg or 250 mcg doses at both vaccination, with 15 people in each dose cohort—says NIH. The first four volunteers will receive an injection with the lower dose, while the next four will receive the 100 mcg dose.
Before the second injection of the vaccination, medical investigators will review the safety data—also before vaccinating the remaining volunteers in the 25 mcg and 100 mcg groups. Another safety review will be conducted before the volunteers are enrolled for the 250 mcg cohort.
But the vaccine will still take 12-18 months before it could be made available on a mass level. The volunteers will be asked to come to the clinic for follow ups between vaccinations and also for additional visits in a span of a year or so.
The vaccine is named as mRNA-1273 and developed by scientists in NIAID, in collaboration with the biotech company Moderna Inc, which is based in Massachusetts, US. The experimental vaccine was developed based on the pattern of the genetic material mRNA (messenger RNA). The SARS-CoV-2 virus is also an RNA virus. The scientists took out part of the virus RNA to produce a specific type of protein. This protein is expected to elicit a strong immune response in the body. The vaccine has reportedly shown effectiveness in animal trials.
mRNA-1273 is not a live vaccine, which means, the doses of injection will not have the live virus, but a part of its genetic material, the RNA. This RNA, when inserted into the body, will produce a viral protein which will bolster the immune system to fight the virus. This is partial deviation from the traditional vaccine development methods and implementation of the modern regime of vaccines where genetic material to produce protein or protein itself are used as catalysts.
Responding to the trials, NIAID director Anthony S Fauci said—“Finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority. This Phase 1 study, launched in record speed, is an important first step toward achieving that goal.”
As COVID-19 has turned into a pandemic, many institutes and scientists have been engaged in this effort, but yet, have not been successful. This new trial, if passes the safety reviews, can bring a hope for the future.
Get the latest reports & analysis with people's perspective on Protests, movements & deep analytical videos, discussions of the current affairs in your Telegram app. Subscribe to NewsClick's Telegram channel & get Real-Time updates on stories, as they get published on our website.