Chemistry Nobel Prize Won by Woman Scientist Only for the Fifth Time in History

Nobel Prize in Chemistry was first awarded in 1901, and since then, 177 people have been honoured with this award. But, when Frances H. Arnold of California Institute of Technology, USA, won the 2018 Chemistry Nobel, she became only the fifth woman to be awarded the prize.
Dr. Arnold, 62, is a professor of chemical engineering, biochemistry and bioengineering won the award as recognition for her work on directed evolution of enzymes.
Dr. Arnold shares the Nobel with George P. Smith of University of Missouri, Columbia, USA, and Georgy P. Winter of the MRC laboratory of molecular biology, Cambridge, UK. Dr. Arnold received half of the prize while Dr. Smith and Dr. Winter split the other half.
‘How to control evolution, and use it for various benefits of humankind’ is the theme of this year’s Chemistry Nobel. The three scientists that won the prize could harness the power of evolution to design molecules having wide range utility. These include enzymes, the biological catalysts, which are produced by controlling evolution in the lab. These enzymes can be used in manufacturing variety of things from biofuels to pharmaceuticals. In a similar way, antibodies are evolved using the method called phage display. These antibodies have the ability to combat autoimmune disease and also in some cases can cure cancer.
The important aspect that the prize highlights is the diminishing of the line between biology and some fields of chemistry. Many chemists have now been turning to biology for better understanding of the nature.
Directed Evolution in Lab
Dr. Arnold conducted the first directed evolution of enzymes back in 1993. Enzymes are proteins that catalyse chemical reaction. Her efforts to remodel enzymes dates back to 1980s and during this early phase of protein engineering she repeatedly failed in her attempts. Soon, she realised that modifying proteins is not easy a task and she turned to evolution.
Proteins are made up of nitrogen containing molecules called amino acids. The amino acids again are directed by the genes in the DNA. The genes direct the way the amino acids are combined in a protein. This combination of amino acids determine the three dimensional structure of a protein. The structural aspects of a protein is what that determines its functions. So, for the remodelling of any protein, the ordering in the amino acids sequences have to be altered. Doing it in the lab is not an easy task to accomplish.
But, nature has its own way to do modification to proteins. Through evolution, the genes get mutated, and for the gene mutation, changes in protein structure and function happen over time. This was the catch point for Dr. Arnold for her attempt of protein rebuilding.
She attempted to modify an enzyme names subtilisin. It was her effort to make subtilisin accelerate changes in an organic solvent. She created random mutation in the enzyme’s genes and introduced those mutated genes to bacteria. The mutated genes in the bacteria got expressed in different forms of subtilisin. Dr. Arnold selected those types that could solve her purpose. She could further mutate it until the finest type is obtained. This is called the directed evolution that won her the Nobel Prize.
Phage Display and Making of Therapeutic Antibodies
Dr. Smith and Dr. Winter developed a slightly different way of protein modification. Dr. Smith was trying to figure out unknown genes that produced known proteins. He took the help of bacteriophages – viruses that infect bacteria. The phages are extremely simpler life forms. They have their genetic element – the DNA and an outer envelope of proteins with few simpler cellular parts. The envelope that covers the DNA is called the capsid of a phage. Dr. Smith put variety of candidate genes into the phages’ DNA. The genes embedded produced proteins that appeared in the outer envelope. Dr. Smith then used antibodies and some phages got attached to these antibodies. The phages got attached to the antibodies applied through the proteins that appeared in the outer coverage of the phage due to the embedded candidate genes. The antibodies were designed to attach to specific proteins.
By finding what antibodies are bound by the envelope protein, Dr. Smith could know what that protein was. From the protein he also could find out the genes that gave rise to the protein. This is called Phage Display.
Figure below describes methods used in phage display:
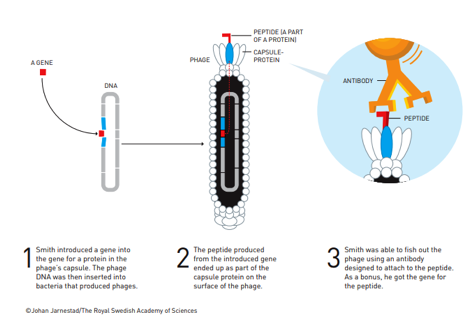
Dr. Winter extended the technique of phage display to develop antibodies having potential of acting as new treatments of complicated diseases like multiple sclerosis and cancer. Dr. Winter inserted genes that can produce antibodies in the phages. He examined the variants of the antibodies and found out the ones that bound most effectively to the desired targets. Eventually, repeated evolution of the gene could bring more effective antibodies.
It was in 2002 that the first antibody drug prepared by this method was approved for human treatment. This antibody drug Adalimumab, is now sold under the brand name of Humira and used for treating rheumatoid arthritis, psoriasis and inflammatory bowel disorders.
There are other antibodies that are in use in killing cancer cells, neutralising anthrax and in autoimmune disease like lupus.
Get the latest reports & analysis with people's perspective on Protests, movements & deep analytical videos, discussions of the current affairs in your Telegram app. Subscribe to NewsClick's Telegram channel & get Real-Time updates on stories, as they get published on our website.















