COVID-19 Treatment: SOLIDARITY Trial is Restarting with 3 Drugs This Time
Image Courtesy: Scincemag.org
The SOLIDARITY trial is an international trial led by the World Health Organisation (WHO), which explores possible drugs that can be repurposed to treat severe COVID-19 patients. Repurposed drugs are those drugs which are already in use in treating other ailments, but can also be effective against COVID-19. The SOLIDARITY trial is the largest global randomised clinical trial conducted over 30 countries.
This trial has already been implemented since the beginning of the pandemic in 2020 in different phases. This time, the SOLIDARITY trial plans to evaluate three drug candidates for their repurposing in the treatment of hospitalised COVID-19 patients.
The three drug candidates are imatinib, which is a cancer drug; infliximab, an antibody and artesunate, an anti-malarial drug.
According to reports, the three drug candidates have already been sent to Finland, which is the first country to have all the approvals cleared. John Arne Rottingen, the person who chairs the study’s executive group and belongs to the Norwegian Institute of Public Health commented:“I expect that the first patients will probably be recruited there any day.”
Other countries are expected to join the trial soon with over 40 nations in the process of clearance of regulatory and ethical approvals.
The first SOLIDARITY trial began in March 2020 and over a dozen of countries are believed to have participated in it to evaluate drug efficacies in treating COVID-19 patients.
In October 2020, the trial produced interim report on four treatments, none of which showed significant benefits. The trial involved 11,000 patients in 400 hospitals in over 30 countries. The four drugs that were taken in the first trial were Remdesivir, an anti-viral drug; Lopinavir/ritonavir combination, which are HIV drugs and Hydroxychloroquine, the anti-malarial drug and interferon Beta.
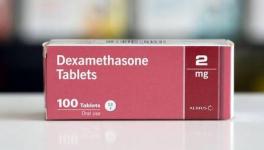
The SOLIDARITY trial is not the only one to assess drug efficacy in COVID-19. There have been other trials, too. For example, the Recovery trial conducted by the United Kingdom could find out two drug candidates effective for treating serious COVID patients.
Dexamethasone, a steroid and a cheap and readily available drug was found to be effective in reducing deaths up to one-third among the participants recruited in the trial. The result came out in June 2020 and dexamethasone has been in use since then.
Again in February this year, the Recovery trial declared another drug to be effective and that is the tocilizumab. Tocilizumab is a monoclonal antibody (a designed antibody) and blocks an inflammatory cytokine molecule called the interleukin-6. Cytokines are protein molecules involved in the immune system (the defence mechanism of the body). Cytokines can cause inflammation in different organs and sometimes these can cause a hyper reactive immune system which may prove dangerous for the body. The cytokine storm (over secretion of cytokines) can cause severe disease in COVID-19 patients.
Tocilizumab was found to reduce mortality in COVID patients. Both Dexamethasone and Tocilizumab are immune suppressants, meaning they can dampen the over reacting immune system in severely ill patients.
The drugs considered in the current SOLIDARITY trial also target the immune system rather than the coronavirus itself.
Imatinib is an oral drug used to treat Leukemia and other types of cancer. Imatinib has been chosen on the basis of a previously conducted trial consisting of 400 hospitalised COVID-19 patients in the Netherlands. The trial results were published in Lancet in June 2021 and showed that patients spent less time on ventilator and mortality was also decreased. Imatinib can protect the alveoli of the lungs, the place where oxygen transfers from the lungs into blood.
Infliximab is an antibody and is administered as a single infusion. This drug is widely used in the treatment of a variety of inflammatory conditions, such as rheumatoid arthritis, inflammatory bowel disease. The basic functioning of this drug is to block tumour necrosis factor (TNF) alpha. TNF alpha is a pivotal protein molecule involved in signaling in the immune system. There have been some observational data suggesting that the drug may be effective in protecting COVID-19 patients with severity of the disease.
Artesunate is used against malaria parasites. This drug has also shown some anti-viral activities in some laboratory studies. However, the main reason behind the recruitment of this drug under SOLIDARITY trial is due to the fact that artesunate can reduce inflammation.
After the first SOLIDARITY trial results came out in October last year, the trial took a long time for its revival. The results of the largest trial with new drugs on board will be awaited eagerly by the medical community.
Get the latest reports & analysis with people's perspective on Protests, movements & deep analytical videos, discussions of the current affairs in your Telegram app. Subscribe to NewsClick's Telegram channel & get Real-Time updates on stories, as they get published on our website.