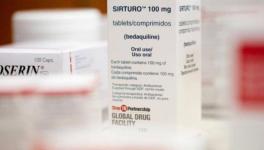
Patent Opposition Filed For Johnson And Johnson’s TB Medicine
Image Coutesy: Down to Earth
Nandita Venkatesan delivered a moving keynote speech in New York last September during United Nations High Level Meeting on tuberculosis. She recounted her experience in the fight against drug-resistant TB (DR-TB). She is a TedX speaker, and has been advocating for better treatment for all the TB patients, including access to new drug bedaquline which is out of reach for the Indian patients. One of the reasons is the monopoly right of the pharmaceutical company Johnson and Johnson, which is not allowing alternate producers to enter the market.
However, things were different for her as a teenager when she learnt about the TB bacteria in her stomach. It was 2007, and she was a 17-year-old college student when she was put on first line TB drugs for 18 months. At that time, she was cured and continued with her normal life. But in May 2013, the disease relapsed, now with the bacteria in its worse drug-resistant form, which meant that she was immune to certain medicines, and had to take those which cause horrific side-effects. She suffered kidney malfunction, memory loss, low sugar and distorted speech.
But the worst nightmare came in November 2013. Venkatesan woke up to a complete silence around her. She had lost her hearing power due to the side-effect of the injectable medicine kanamycin.
“I have gone through the worst side effects of DR-TB. I have spent nights and days crying out of pain. I know what a patient goes through. New medicines were not available during my treatment. But now they are, and all the patients should have access to them. That’s why I am challenging extension of monopoly of bedaquiline,” said Venkatesan.
Bedaquiline is a new DR-TB drug. It was the first TB drug to be discovered after a gap of 50 years. It has certain benefits – it comes in tablet form, and hence can be taken orally, making it easy for patients to consume. It is a new medicine and patients are not yet resistant to it. It is a safe and effective drug if given under proper supervision. In its latest guidelines for treatment of DR-TB released in December 2018, the World Health Organisation (WHO) has included bedaquiline as a core drug for DR-TB treatment, and highlighted the need for “immediate steps to be taken to ensure that MDR/RR-TB patients receive treatment in accordance with the latest evidence on effectiveness and safety”. It can also be used to treat children aged six years and above, which is very important because it is more difficult for them to bear with the side-effects of the older medicines. South Africa has already started using the drug for all patients with DR-TB, based on the safety data that the country has generated.
However, lack of alternate producers is acting as a disincentive to scale up bedaquiline by the governments of the countries with high burden of TB and DR-TB such as India. A six-month-long course of bedaquiline costs US$ 400 or nearly Rs 28,000. This is just one of the drugs from a combination of four to five antibiotics needed to treat DR-TB. The main barrier to affordable cost is the benefits of a patent that Johnson and Johnson (J&J) enjoys. According to a study by Prof. Andrew Hill of University of Liverpool, United Kingdom, bedaquiline would cost US$ 8 to 17 per month if produced by generic manufacturers, of which there is no dearth in India. For a six-month course, the cost would range between US$ 48 to 102, which is nearly Rs 3400 to 7200. Thus, there can be a substantial drop in prices once generic production kicks in.
The company is yet to produce child-friendly version and its patent is not allowing other manufacturers to start producing it.
So far, the Indian government has depended on donations from the company to treat the patients. As the donation expires in March this year, it is going to cause a huge cost on exchequer of India’s health budget.
The current patent in India is on the compound and gives monopoly to J&J till year 2023.
What Nandita and her co-petitioner from South Africa, Phumeza Tisile, are challenging is a further extension of the patent that J&J has applied for the salt form of the drug. If granted, the monopoly of the company will extend till December 2027. It is called evergreening, and is not permissible by the Indian law.
“A composition of known compounds that does not deliver any improved effect cannot be granted a patent. The claim of this application is such a composition, combining known exepients with bedaquiline salt,” said Priyam Lizmary Cherian, a lawyer with Lawyers Collective, lawyer of one of the petitioners.
J&J has claimed the patent for a bedaquiline salt formulation which is an old technique and a known technique. It is interesting that the company copied and pasted the recipe from one of its own old patent application for HIV drug Rilpivirine that was rejected by the patent office. This shows that the salt formulation is not new even for J&J, and they have used it before to produce drugs.
Tisile has been fighting patents for a long time as part of Fix the Patent Law campaign in South Africa. “I never fully understood the logic behind making drugs that people have no access to because of the higher prices. It’s common knowledge that TB is the disease of the poor, and in most cases, people don’t have access to life-saving drugs. The aim of this patent challenge is to have life-saving drugs for all,” said Tisile. She said that reducing the price of TB medicines in India will have a positive effect on patients in South Africa and other developing countries too.
Tisile too, like Venkatesan, lost her hearing to kanamycin when she was 19 years old. She was diagnosed with DR-TB in 2010, and within months of starting her treatment, she realised that she could not hear anything. After a gruelling treatment that went on till 2013, Tisile recovered from the disease and became one of the leading advocates for TB patients.
Both Venkatesan and Tisile have recovered partial hearing due to cochlear implant. But it has cost them a fortune. Venkatesan’s implant cost her Rs 27 lakhs, for which she received help from well-wishers.
“My TB treatment had cost me Rs 40 lakhs, as I was treated in private hospitals and had to endure six surgeries. My parents had sold our house and shifted to a rented accommodation,” says Venkatesan, whose father is a chartered accountant and mother is a teacher. “We had no money left for cochlear implant.”
Both these women want new drugs to be available to all deserving patients so that they do not go through the same ordeal of suffering side-effects.
Jyotsna Singh is a Delhi-based journalist who also writes about health.
Get the latest reports & analysis with people's perspective on Protests, movements & deep analytical videos, discussions of the current affairs in your Telegram app. Subscribe to NewsClick's Telegram channel & get Real-Time updates on stories, as they get published on our website.