COVID: New Hope Rides on Protein-Based Vaccines
Novavax has applied for approval in the EU and other protein-based vaccines are expected to follow
Many people who refuse to get vaccinated against COVID-19 say they don't trust the new technology behind mRNA vaccines, such as the BioNTech-Pfizer and Moderna jabs. They say they also don't trust vector-based vaccines, like the Oxford-AstraZeneca and Johnson & Johnson jabs.
Instead, they say they are waiting for protein-based vaccines, which have proved themselves over years as a safe protection against influenza, tetanus and whooping cough.
At time of writing, the European Union was expected to approve the first protein-based vaccines against the coronavirus SARS-CoV-2.
Protein-based vaccines appear to offer a good level of protection against COVID-19 and produce fewer side-effects than those existing and approved mRNA and vector-based vaccines.
Urgently needed for global vaccine campaign
Experts say protein-based vaccines are urgently needed for the global vaccination program against COVID-19. They say that while many richer nations get busy boosting their populations with a third jab, we often forget that many people in poorer nations have yet to get vaccinated at all.
While rich nations mount booster vaccination campaigns, poorer countries have yet to get basic protection.
Researchers say protein-based vaccines could help people in poorer nations get vaccinated. Protein-based vaccines are cheaper to produce than mRNA vaccines and they can be stored at temperatures of 2-8 degrees Celsius, which makes them easier to transport. So, it would be practical to deploy them in the so-called "global South."
Promising candidates
It's taken longer to develop protein-based corona vaccines. It wasn't until November 2021 that the US pharmaceutical firm Novavax submitted its approval application to the European Medicines Agency (EMA). It's expected that the US will approve the vaccine for use there by the end of the year.
Indonesia gave the Novavax COVID-19 vaccine emergency approval at the start of November. Submissions for approval are ongoing in the United Kingdom, Canada and Australia.
Novavax may well be ahead in the approval stakes. But other vaccine developers, such as India's Biological E and China's Clover Biopharmaceuticals are expected to submit their own protein-based candidate vaccines for approval as well.
Then there's the British-French company, Sanofi-GlaxoSmithKline, a Canadian firm called Medicago and a South Korean one called sk bioscience. Each one continues to progress with their development of protein-based vaccines.
But protein-based vaccines are produced in many more countries. In some, such as Cuba, Russia and Taiwan, protein-based vaccines are a standard in national vaccine campaigns.

Not all people unvaccinated against COVID-19 are fundamentally against vaccines — they just don't trust the newer technologies in existing vaccines.
How do protein-based vaccines differ?
Protein-based vaccines include a tiny, look-alike version of the COVID-19 spike protein.
The immune system reacts to the protein in the vaccine and it does that a lot faster because — contrary to other vaccines — it doesn't have to produce the protein itself. The protein is delivered with the vaccines.
Novavax does not contain any killed coronavirus. Instead, the developers used a recombinant nanotechnology to generate the tiniest particles that resemble the SARS-CoV-2 spike protein.
Using insect cells, the developers created a nanoparticle that the body's immune system recognizes as the virus ("a virus-like particle") — although it is not the virus — and reacts accordingly.
Those nanoparticles do not carry any DNA of the virus and therefore produce fewer side effects in the human body. But the human immune response is weaker.
Stronger effect with adjuvants
To strengthen the immune response, the developers add so-called adjuvants to these vaccines. In the case of the Novavax vaccine, the adjuvant is saponin (extracted from Quillaja saponaria Molina bark) and cholesterol and phospholipids.
Some vaccine critics say some adjuvants, such aluminum salt, are harmful.
But meta-studies have so far failed to find any links between such adjuvants and serious side-effects or allergies.
Long development
Before mRNA vaccines got their long-awaited break in the COVID pandemic, protein-based vaccines were considered a future-oriented, tried and tested technology.
That's the view of Carlos Guzman, director of the department of Vaccinology and Applied Microbiology at the Helmholtz Centre for Infection Research and a professor at the Hannover Medical School.
"Protein-based vaccines are just very well-known, people's bodies tend to tolerate them better [than other vaccines], and there are no big questions left to answer," says Guzman. "But one disadvantage is that it takes longer to develop protein-based vaccines than mRNA or vector-based vaccines."
How effective are protein-based vaccines?
It may take longer to develop them, but protein-based vaccines are cheaper to make, easier to transport and effective as well.
Novavax says its vaccine has an efficacy of 90.4%. That puts it in the same league as the mRNA vaccines from BioNTech-Pfizer and Moderna.
But that efficacy rate was taken from studies in mid-2021 in the USA and Mexico.
A British study, meanwhile, conducted when the alpha variant of the coronavirus was dominant in the UK, showed the Novavax vaccine to have an efficacy of 83%.
And a study conducted around the same time in South Africa, where the beta variant was dominant, the Novavax vaccine scored an efficacy of just 50%.
If newer variants, such as delta and omicron, keep showing themselves to be more and more transmissible, then it's possible that all vaccines will see a drop in their efficacy — the protection they offer people.
That's why we all need booster jabs. But at the same time we cannot afford to forget the poorer nations, struggling with their first and/or second jabs.
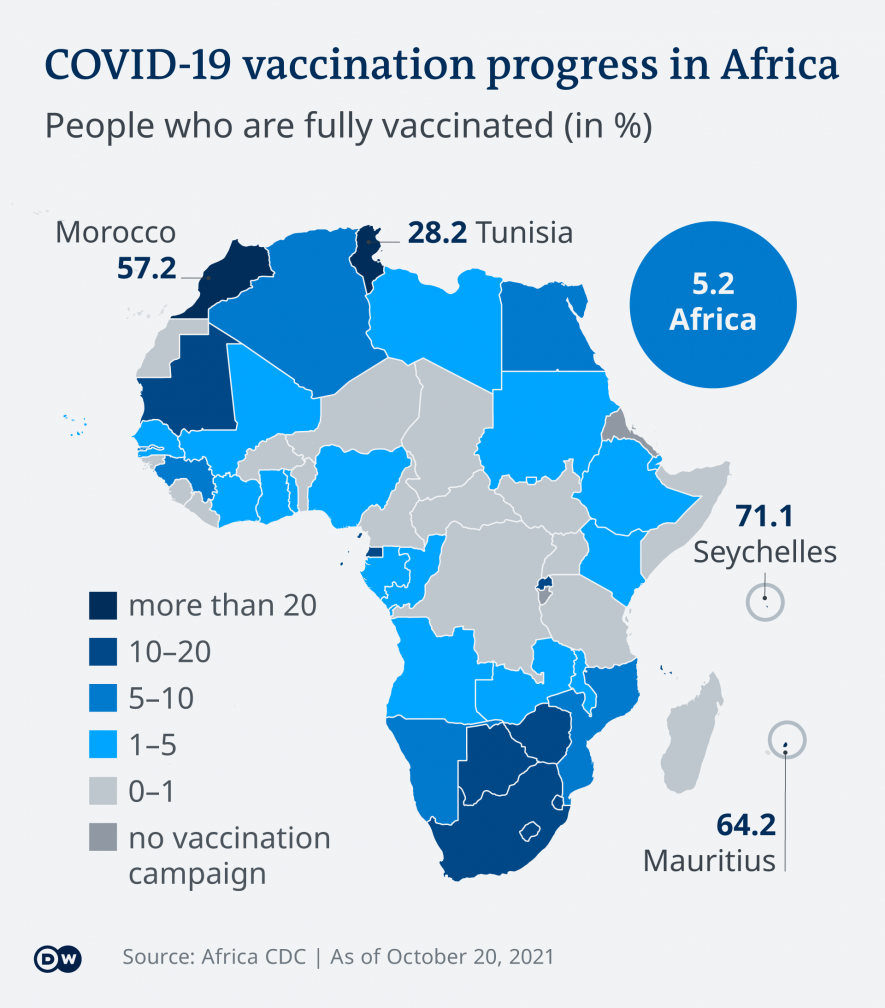
The global vaccination campaign
Next to existing COVID vaccines, many millions of doses of which have been pledged to poorer nations through the COVOX program, protein-based vaccines will no doubt be used there as well — once they are approved for use.
Novavax has pledged a billion doses through COVAX.
The company says it could produce 100 million doses — possibly even 150 million doses — per month.
Its chief executive, Stanley Erck, has said that many of the first doses they produce will go to poorer nations. That was always their goal, he says.
This article was originally written and published in German on 26.11.2021. It was abridged and translated by Zulfikar Abbany on 17.12.2021. The English text was edited by Fabian Schmidt.
Get the latest reports & analysis with people's perspective on Protests, movements & deep analytical videos, discussions of the current affairs in your Telegram app. Subscribe to NewsClick's Telegram channel & get Real-Time updates on stories, as they get published on our website.