UK Approves First Pill to Treat Covid-19
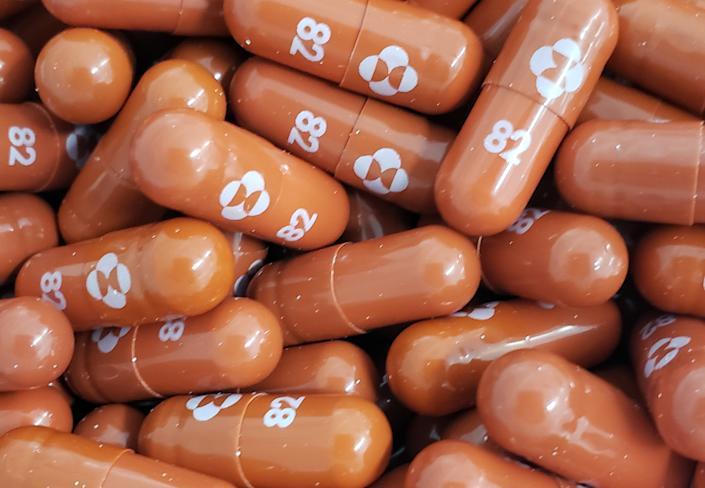
Image Courtesy: Reuters
The UK medicines regulator, Medicines and Healthcare products Regulatory Agency (MHRA), has approved the first pill designed to treat symptomatic COVID cases.
The tablet - molnupiravir - was initially developed to treat flu by the US drug companies Merck, Sharp and Dohme (MSD) and Ridgeback Biotherapeutics. It would be given twice a day to vulnerable patients recently diagnosed with COVID-19. The clinical trials of the pill showed that it cut the risk of hospitalisation or death by about half.
UK Health Secretary Sajid Javid hailed the pill as a "gamechanger" for most frail and immunosuppressed patients.
"Today is a historic day for our country, as the UK is now the first country in the world to approve an antiviral that can be taken at home for COVID," Javid said.
Molnupiravir is the first antiviral COVID medication that can be consumed as a pill. Until now, every medication was either injected or given intravenously. The UK has ordered 480,000 courses of the drug. The first deliveries are expected in November.
Although the UK has not disclosed the cost of its initial contract for 480,000 courses of molnupiravir, the US authorities had recently placed an advance order for 1.7 million courses worth $1.2 billion, priced at $700 for each patient.
The UK government's initial plan includes giving the pill "to both vaccinated and unvaccinated patients through a national study, with extra data on its effectiveness collected before any decision to order more." The medication requires to be given "within five days of symptoms developing to be most effective."
The new treatment stops the COVID virus from multiplying in the body by attacking an enzyme that the virus uses to produce copies of itself. The treatment helps in "keeping virus levels low in the body and reducing the severity of the disease."
Merck, one of the developers of this pill, said that this particular approach of the drug in attacking virus' multiplying tendencies should make it "equally effective against new variants of the virus as it evolves in the future."
The UK medicines regulator said the tablet had been approved for use in people having mild to moderate COVID effects and "at least one risk factor for developing severe illness such as obesity, old age, diabetes or heart disease."
The MHRA's chief executive, June Raine, described the pill as "another therapeutic to add to our armoury against COVID-19."
"It is the world's first approved antiviral for this disease that can be taken by mouth rather than administered intravenously," she said.
In the clinical trials conducted on 775 non-hospitalised patients, the drug "was shown in lab studies to disrupt the ability of the SARS-CoV-2 virus to replicate its genome. SARS-CoV-2 is the coronavirus that causes COVID-19."
Data from the trials indicate that molnupiravir should be taken soon after symptoms emerge to have an effect. A similar thing is said by the MHRA in its approval note, which recommends that the drug be used "as soon as possible" after a positive COVID test and within five days of symptoms onset.
Get the latest reports & analysis with people's perspective on Protests, movements & deep analytical videos, discussions of the current affairs in your Telegram app. Subscribe to NewsClick's Telegram channel & get Real-Time updates on stories, as they get published on our website.