Drug-Resistant TB: Accessibility of Drugs Key in Eradication, Writes Kerala MP
Image for representational use only.Image Courtesy : BI
T N Prathapan, Congress MP from Thrissur constituency of Kerala, wrote a letter to Prime Minister Narendra Modi on Friday, February 14, urging him to take actions to scale up the treatment for Multi-Drug Resistant Tuberculosis (MDR-TB) and Extensively Drug-Resistant Tuberculosis (XDR-TB). Despite being one of the countries with the highest TB burdens in the world, a major section of India’s population does not have access to the drugs required for treatment of the disease.
In a written answer to Unstarred Question 1023 asked in the Lok Sabha, the MoS in the Ministry of Health and Family Welfare (MoHFW), Ashwini Kumar Choubey on February 7, 2020, pointed out that according to the data collated by the NIKSHAY portal, 97,29,822 cases of TB were reported between 2015 and 2019. The number of reported cases has seen a constant rise, from 16,09,278 reported cases in 2015, to 23,82,004 reported cases in 2019, showing that the number of cases going unreported has certainly decreased.
In July 2019, the MoHFW had signed a memorandum of understanding (MoU) with the Ministries of Defence, Railways, and AYUSH with the ambitious goal of eradicating tuberculosis from India by 2015. According to the Global Tuberculosis Report 2018 published by the World Health Organisation (WHO), two of the primary routes to reducing TB incidence and death are diagnosis and treatment, areas where “large and persistent” gaps remain.
Of the 10 million new and relapsed cases in 2017, only 6.4 million, that is, 64%, were officially reported to national authorities and the WHO. Ten countries accounted for 80% of the 3.6 million gap, led by India (26%), Indonesia (11%), Nigeria (9%). The gap was suspected to be caused by a combination of under-diagnosis and under-reporting. India also led in cases of Multi-Drug Resistant TB (MDR-TB). Nearly half of the world’s MDR-TB cases were in India, according to the WHO report. According to the answer of the Unstarred Question 3025 asked in Lok Sabha, Choubey said on December 6, 2019 that 1,30,000 cases of drug-resistant TB were reported in the country in 2018.
He said in the answer, “[I]n absolute numbers, India has the largest number of individuals suffering from drug resistant TB. However, as per the latest global TB report, India ranks 38th in the number of MDR-TB patients per lakh population (9.6 per lakh population). The estimated number of Multi drug resistant/ Rifampicin resistant TB (MDR/RR TB) patients in India for 2018 is 1,30,000, which contributes to about 27% of the global burden.”
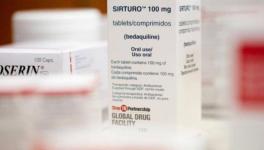
According to the letter written by Prathapan, in 2015, WHO included two new drugs in the treatment of drug-resistant TB—Bedaquiline and Delamanid—in its ‘Model List of Essential Medicines’. Following this, the Central Drug Standard Control Organisation (CDSCO) approved access for MDR/XDR-TB through the Revised National Tuberculosis Control Program (RNTCP). Delamanid was also approved by the CDSCO for children and adolescents aged 6-17 years with drug-resistant TB. However, these medicines have still not been included in India’s National List of Essential Medicines (NLEM).
Since Bedaquiline and Delamanid are under patent monopoly, there is no generic availability of these lifesaving medicines in the country. Instead of taking steps to start domestic generic production of these essential medicines, RNTCP is dependent on donations, and ‘charity pricing’ from the patent-holding companies to treat patients. The letter says, “Given India is amongst the highest TB burden countries in the world, the quantities of Bedaquiline and Delamanid donated to the RNTCP are grossly inadequate to treat the drug-resistant TB population in India.”
The requirement of these two drugs will increase with increased availability of drug sensitivity testing (DST) of drug-resistant TB patients under the RNTCP program. The absence of the required number of doses will result in worsening of MDR/RR-TB treatment and risk of failure resistance. According to the letter, “Once the donation programme is over, the government would have to purchase Bedaquiline and Delamanid at high prices.” Bedaquiline is priced at about Rs 28,567 for a six-month course, and Delamanid at Rs 1,21,413 for a six-month course.
Prathapan has requested the prime minister to ensure that there is no unmet demand for these two new medicines and all patients requiring these medicines are treated properly. He has also urged the PM to direct the Health Ministry to initiate steps to include both these drugs in NLEM, and to put an end to the country’s dependency on donation of drugs for treatment of TB.
Get the latest reports & analysis with people's perspective on Protests, movements & deep analytical videos, discussions of the current affairs in your Telegram app. Subscribe to NewsClick's Telegram channel & get Real-Time updates on stories, as they get published on our website.