What is ‘Click Chemistry’ That Won Nobel This Year?
Image Courtesy: nobelprize.org
What do we do when we put a seat belt into the buckle with the familiar sound of a ‘click’? We lock the seat belt into the buckle. We do this quite at ease and without paying much attention. Can chemistry be this simple?
On the one hand, there is one molecular entity, and on the other hand, there is another, and they can be locked just like the belt and the buckle? Indeed, chemists have made locking molecules that simple, and it is more than imagination or a distant possibility; scientists have won the Nobel prize for it. Not only that, but moving further, the ‘click chemistry’ has been applied inside human cells, and they are used in mapping how cells function. This sums up the Nobel Prize in Chemistry for the year 2022—it's just a click, and molecules get coupled together.
Barry Sharpless, Scripps Research, USA; Morten Meldal, University of Copenhagen, Denmark and Carolyn Bertozzi, Stanford University, USA, have been jointly conferred with the Chemistry Nobel this year.
Modern chemistry spurred researchers to construct naturally available molecular structures in their laboratories. To imitate the stunning molecular structures in nature was indeed fascinating research during the youth of modern chemistry, precisely in the eighteenth century. This drive of research also resulted in the development of important pharmaceuticals. To construct the natural molecules in the laboratory test tubes was proven worthy over time, and chemists kept rolling on it, acquiring the ability to create amazing molecules with sophisticated tools.
However, there has always been a 'but' that chased the world of chemistry, and that is the complexity involved in constructing natural molecules in laboratories. Complex molecules often involve multiple steps, which is not only time-consuming and expensive but also results in generating unwanted by-products. Removal of the by-products is pertinent and involves a substantial loss of material.
Creating something in this process in a minuscule amount is still okay, but chemists are challenged overwhelmingly when it comes to large-scale production. The 2022 Nobel prize is actually making things simpler where molecules can bind together in the desired fashion quickly and efficiently as well.
This necessity moved many chemists to find a simpler and more efficient way of constructing molecules. Making something simple can be more complex and challenging sometimes; however, chemists did not cease to accept it and found a new innovator in the form of Barry Sharpless, who won this year's Nobel, his second time till now. During the early 2000s, Sharpless coined the concept of ‘Click Chemistry’ wherein molecular building blocks can bind together quickly and efficiently.
Barry Sharpless's efforts got a boost when Morten Meldal, the other Nobel winner this year, independently discovered one of the most important aspects in click chemistry and, for that matter, chemistry as a whole, which is the 'copper-catalysed azide-alkyne cycloaddition.'
In his early review paper published in 2001, Sharpless argued that chemistry has come to the juncture where it has to move towards simpler reactions, where the unwanted by-products are minimalised. He argued that just a few good reactions are enough to assemble a vast number of diverse organic molecules. In his new concept of click chemistry, Sharpless found new ways to design pairs of molecules that can react only with each other and that too irreversibly.
COPPER-CATALYSED AZIDE-ALKYNE CYCLOADDITION
Azides are anions (negatively charged ions) consisting of three double-bonded nitrogen atoms. They typically exist as a functional group. On the other hand, Alkynes are molecules containing a triple bond between two carbon atoms. Chemical bonds are extremely important that bind atoms together in a molecule. Double and triple bonds are chemical bonds holding different atoms in a molecule.
Azides and alkynes can react very efficiently when copper ions are added. This reaction has turned chemistry to a great extent and is used globally to snap molecules together. In an illustration, the Nobel Prize website has shown the following:
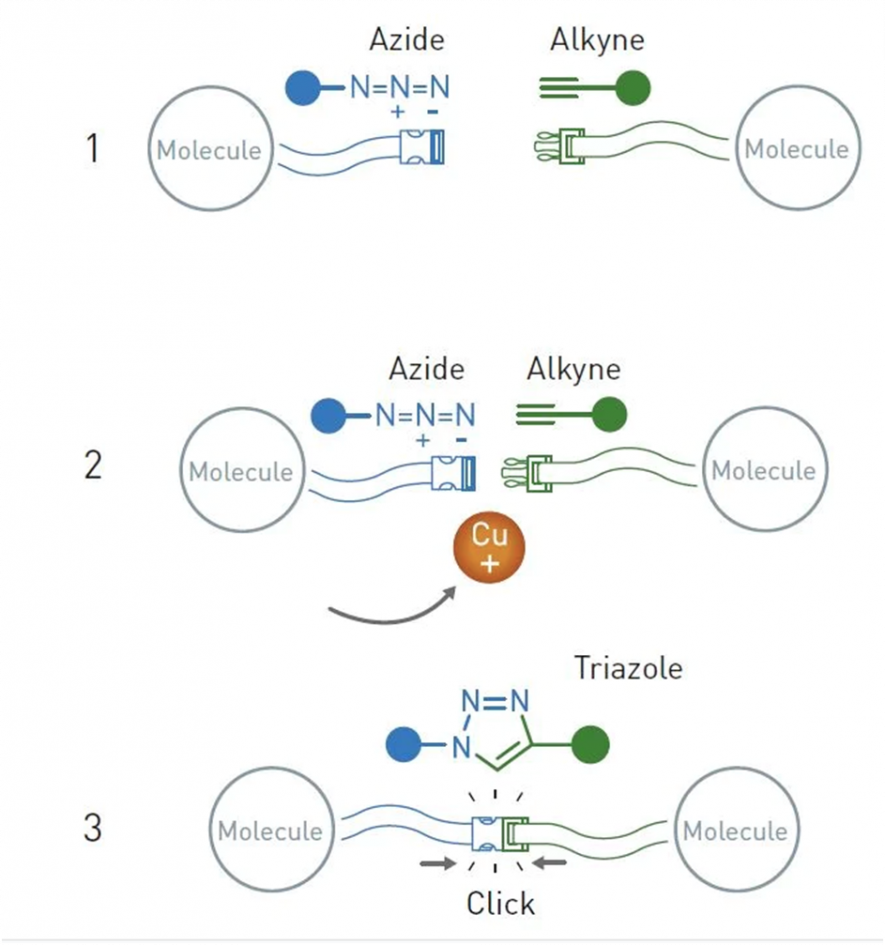
Image source: nobelprize.org
When azides and alkynes react, they produce traizoles, an important chemical building block found in pharmaceuticals, dyes, and agricultural chemicals. Scientists previously tried this reaction, but they also produced unwanted by-products. Morten Meldal found that copper ions added to the reaction system control it, and only one substance has been formed. Meldal first produced his results at a symposium in San Diego in the year 2001, and the next year he came out with a publication where he showed how this reaction could be used to bind various other molecules.
Apart from Meldal, Barry Sharpless also published a paper independently showing the copper-catalysed azide-alkyne reaction in water and its reliability. According to Sharpless, it is the perfect click reaction.
As seen in the figure above, any two molecules chemists want to link together can now easily be done by introducing one molecule to the azide and the other to the alkyne and letting the copper-catalysed click reaction run on.
This simple reaction made the construction of many complex molecules easier in research labs and industrial applications. The importance of it is that the click reaction can facilitate the production of newer materials for specific purposes. Since its discovery, the click reaction has been used for manufacturing new materials.
‘CLICK CHEMISTRY’ AND HUMAN CELLS
So, what about using it inside human cells? As the click reaction involves only the reactants and does not, as such, touch other materials present in the reaction beaker, then using it inside cells will ensure that only the desired materials get involved, and the rest of the cellular processes will remain unhurt. This could be an incredible idea in the treatment of complex diseases like cancer.
Carolyn Bertozzi, the third winner of the Nobel this year, followed how the click reaction could be used for cellular purposes. Around the same time, Bertozzi was busy studying glycans, which are complex sugars found on the surface of bacteria. Bertozzi was into mapping the glycans, which was a remarkably difficult task during that time.
In the year 2000, Bertozzi found an optimal chemical handle in the form of azide. She, in her valuable addition to biochemistry, linked a fluorescent molecule to the azide, which was, in turn, introduced to the glycans. Importantly, azides don't affect the cells, and apparently, there is no harm in applying them, even in living creatures. At that time, the click chemistry of Sharpless and Meldal was spreading among chemists. But for Bertozzi to use it as such in a cell was a problem because the copper ions involved in the click reaction can be toxic to the cells.
Bertozzi was quick on her spree to find an alternative to copper to carry the azide-alkyne reaction in cells. Finally, in 2004, Bertozzi published the click reaction without copper ions.
Bertozzi hereafter went ahead to further refine her click reaction to use in a better way in a cellular environment. Along with her, many other biochemists explored how biomolecules can interact in cells.
Bertozzi also studies the glycans present on the tumour cells' surface. Her studies found that in some cases, the glycans on the tumour cells protect them from an attack of the immune system (the immune system not only fights invading agents, it also fights tumours inside the body). She and her colleagues have been able to create a new type of pharmaceutical that can break down the glycans present on the tumour cell surface. This pharmaceutical is now in clinical trials on advanced cancer patients.
In addition, many researchers have developed antibodies that can target tumour cells with the help of the click reaction. After the antibody is attached to a tumour, a second molecule that can click to the antibody is injected into it. The world is yet to see the potential of click chemistry and its application to new pharmaceuticals and new therapies; however, it is sure that the game has been started.
Get the latest reports & analysis with people's perspective on Protests, movements & deep analytical videos, discussions of the current affairs in your Telegram app. Subscribe to NewsClick's Telegram channel & get Real-Time updates on stories, as they get published on our website.